In July 16, 2024, Neuron published the latest research by Professor Shumin Duan and Yanqin Yu's team from the School of Brain Science and Brain Medicine at Zhejiang University. The article, entitled “A Hypothalamic-Amygdala Circuit Underlying Sexually Dimorphic Aggression,” identifies a hypothalamic-amygdala circuit that mediates male-biased aggression in mice.
Mammals have evolved sexual reproduction to achieve higher evolutionary potential and adapt to environmental changes, resulting in an efficient division of labor. Many instinctive behaviors are sexually dimorphic to enhance survival and reproductive capabilities. Aggressive behavior, an innate behavior, is a powerful tool for guarding territories, competing for critical resources, and defending oneself and family. It is more prevalent in males due to selective pressures associated with limited mating opportunities, with males typically exhibiting higher levels of aggression. Sexually dimorphic aggression is a stereotypical innate behavior with evolutionary conservation, genetically hardwired to be displayed without training. While brain areas are known to elicit sexually dimorphic or monomorphic aggression in rodents, how these circuits are interconnected and gate sexually dimorphic attacks remain unclear.
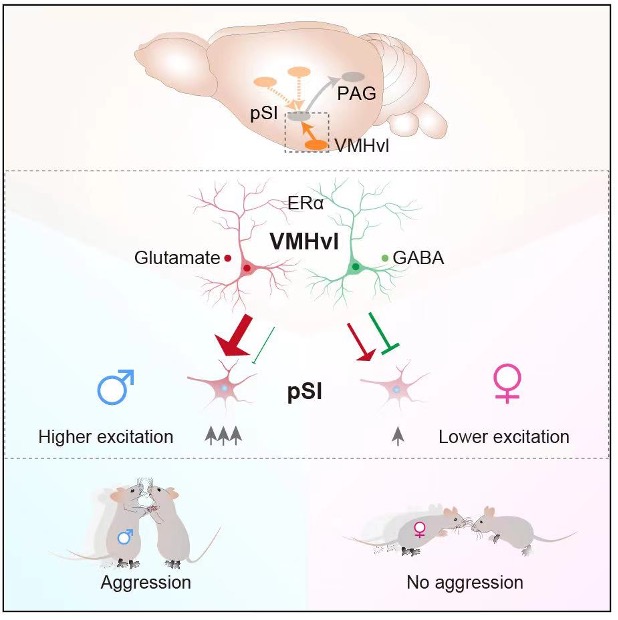
Decades of studies have identified the ventrolateral part of the ventromedial hypothalamus (VMHvl) as a key region associated with male-biased aggression. In this study, the team screened cFos expression in downstream brain regions when chemogenetic activation of estrogen receptor-α (Esr1) positive VMHvl neurons in male and female mice, identifying a potential downstream target in the posterior substantia innominata (pSI). The pSI, an area in the extended amygdala, promotes similar levels of attack in both sexes of mice.
Anterograde and retrograde tracing revealed that the VMHvl sends projection terminals to the pSI. Optogenetic inhibition of pSI neurons during chemogenetic activation of VMHvlEsr1 neurons confirmed that the VMHvl functionally innervates the pSI unidirectionally. The study showed that while excitatory neurons in the VMHvl promote sexually dimorphic aggression, the role of inhibitory VMHvl neurons in regulating male and female aggression is less understood. The team found that the VMHvl innervates the pSI through both excitatory and inhibitory connections. In males, strengthened excitatory VMHvl-pSI projections promote aggression, whereas stronger inhibitory connections in females reduce aggressive behavior. Overall, the convergent hypothalamic input onto the pSI leads to heightened pSI activity in males, resulting in male-biased aggression when VMHvlEsr1-pSI projections are opto-activated.
In conclusion, these studies suggest that a convergent, sexually distinct circuit from the VMHvl to the pSI mediates male-biased aggression. The sexually distinct excitation-inhibition balance of the hypothalamic-amygdala circuit underlies sexually dimorphic aggression.
Drs. Zhenggang Zhu and Lu Miao from the School of Brain Science and Brain Medicine of Zhejiang University are co-first authors. Professors Shumin Duan and Yan-Qin Yu from the School of Brain Science and Brain Medicine of Zhejiang University are the corresponding authors. This work was supported by grants from the National Natural Science Foundation (NSFC) of China (82288101, 82090033, U20A6005; T2293733, T2293730; 32171007; 32241004); STI2030-Major Projects (2021ZD0203400); Key R&D Program of Zhejiang Province (2024SSYS0017,2020C03009; 2022C03034); CAMS Innovation Fund for Medical Sciences (2019-I2M-5-057); the Natural Science Foundation of Zhejiang Province (LZ22H090001); the Non-profit Central Research Institute Fund of Chinese Academy 451 of Medical Sciences (2023-PT310-01).




 Location :
Location : 

