The research team led by Prof. Hao Wang has recently published an article titled “Neural adaption in midbrain GABAergic cells contributes to high-fat-diet induced obesity” in Science Advances.
In modern society, high-calorie food is readily available and easily accessible, leading to a steady increase in the incidence of obesity. Obesity, in turn, contributes to the rise of other related diseases such as hypertension, hyperlipidemia, and diabetes, placing a significant burden to both society and families. Consequently, obesity has emerged as a pressing public health concern worldwide, with limited treatment options.
While previous studies have demonstrated that weight control can be achieved through modifications in dietary structure and lifestyle habits, it is often observed that individuals in this population tend to regain weight within five years. This phenomenon may be attributed to the impact of high-calorie foods, which not only influence body weight and metabolism, but also induce irreversible changes in the central nervous system. To delve deeper into this issue, Professor Hao Wang and his team from Zhejiang University’s School of Brain Science and Brain Medicine conducted a research study entitled “Neural adaption in midbrain GABAergic cells contributes to high-fat-diet induced obesity”, which was published in Science Advances. The study discussed the neural adaptations observed in midbrain GABAergic cells as a result of high-fat-diet (HFD) induced obesity.
Professor Hao Wang's research team has been dedicated to investigating the neural circuit mechanisms involved in regulating energy metabolic homeostasis. In their previous work published in Cell Reports (2019), they made a significant discovery that GABAergic neurons in the ventrolateral periaqueductal grey (vlPAG) region possess an appetite-suppressing effect.
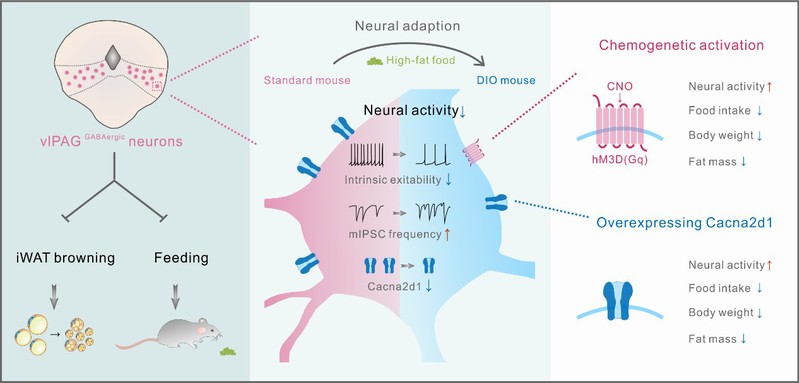
In their current study, the team utilized a chemogenetic approach to activate vlPAG GABAergic neurons and observed a reversal of the obesity phenotype in high-fat-diet-induced obese (DIO) mice. This rescue effect was achieved by reducing 24-hour food intake, increasing energy metabolism levels, and inducing browning of adipose tissue.
Through the use of in vivo fiber-photometry calcium imaging, the researchers discovered that calcium signals originating from vlPAG GABAergic neurons are suppressed during food intake. Notably, these neurons exhibited stronger suppression in DIO mice. Further electrophysiological recordings provided insights into the mechanisms underlying these observations. The reduced excitability of the "food-suppressed" neurons in obese mice was found to be a result of increased presynaptic inhibitory inputs and a decrease in intrinsic excitability of the neurons themselves. Consequently, chronic high-fat food intake leads to long-term inhibition of these "food-suppressor" neurons, ultimately contributing to increased food intake and obesity.
The team further employed single-cell nuclear transcriptome sequencing technology to conduct a comprehensive analysis of gene expression changes in GABAergic neurons within the vlPAG of obese mice, comparing them with control mice. They identified a crucial gene called CACNA2D1, which exhibited significantly reduced expression levels in obese mice. To investigate the potential role of CACNA2D1, the team performed AAV-overexpression of CACNA2D1 in the vlPAG of obese mice. Remarkably, this intervention led to the rescue of the obesity phenotype observed in DIO mice. The rescue effect was achieved by reducing food intake and promoting adipose tissue browning. Additionally, the restoration of CACNA2D1 expression resulted in the recovery of excitability in the "food-suppressor" neurons located in the vlPAG. These findings suggest that CACNA2D1 holds promise as a potential target for the treatment of stubborn obesity.
In summary, Prof. Hao Wang's team found that the "food-suppressor" neurons in the vlPAG are involved in the regulation of energy balance and help maintain body weight homeostasis. However, long-term high-fat food intake will cause these "food-suppressor" neurons to go on strike, which makes the animals unable to stop eating high-fat food, and the vicious cycle of excessive food intake will continue. CACNA2D1 may be a potential target for the treatment of recalcitrant obesity.
GABAergic neurons in the periaqueductal grey regulate weight metabolic homeostasis.
Professor Hao Wang from the School of Brain Science and Brain Medicine at Zhejiang University served as the corresponding author, while Dr. Xiaomeng Wang and Dr. Xiaotong Wu were the co-first authors of this paper. The study received support from Professor Shumin Duan, Professor Xiaoming Li, Professor Yudong Zhou, Professor Chen Li, Professor Han Xu, Professor Jiadong Chen, Professor Wei Gong, Professor Fang Guo, and Professor Ruimao Zheng. Additionally, Dr. Bingwei Wang, along with PhD students Hao Wu and Hanyang Xiao, made significant contributions to this research. Funding for this study was provided by the National Natural Science Foundation of China and Boehringer Ingelheim in Germany.




 Location :
Location : 

